

Discussion forum for Pharma Quality events, Regulatory Actions
Warning letters, 483s, Recalls, Import Alerts, Audit observations

Warning letters, 483s, Recalls, Import Alerts, Audit observations

The USFDA guidance gives a framework for predicting the mutagenic and carcinogenic potential of NDSRIs and recommend acceptable intakes (AI). NDSRIs share structural similarity to the API, are unique to specific APIs and generally formed by nitrosation of API / API fragments that have secondary of tertiary amine structural elements. NDSRIs mostly lack carcinogenicity and mutagenicity study data and an AI limit is not determined for most NDSRIs.
The guidance gives a framework of Predicted Carcinogenic Potency Categorization (PCPC) approach based on structure-activity relationship (SAR) for predicting the carcinogenic potential of NDSRIs. The prediction is based on structural environment surrounding the N-nitroso group of the NDSRI. The categorization, based on the NDSRI’s activating and deactivating structural features helps to assign a recommended AI limit to an NDSRI. The approach assumes that the α-hydroxylation mechanism of metabolic activation is responsible for the mutagenic and carcinogenic response of an NDSRI. The approach applies to NDSRIs bearing a carbon atom on both sides of the N-nitroso group; it does not apply to NDSRIs where the N-nitroso group is within an aromatic ring.
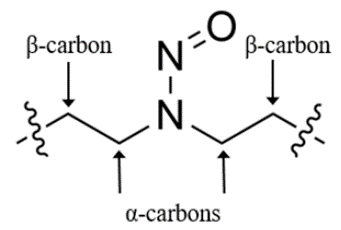
Five potency categories are defined based on number of Hydrogens on alpha carbons, Deactivating features and Activating features with Potency Category 1 assigned lowest AI of 26.5ng/day and Category 5 assigned highest AI of 1500ng/day. The predicted carcinogenic potency categorization approach enables manufacturers and applicants to identify the appropriate potency category and associated recommended AI limits for NDSRIs in APIs and drug products. However the predicted AI should not be applied to NDSRIs where FDA has otherwise recommended an AI limit, based on compound-specific assessments.
| Potency Category | Recommended AI (ng/day) (USFDA Guidance) | Recommended AI (ng/day) (EMA Q&A Document) |
| Category 1 | 26.5 | 18 |
| Category 2 | 100 | 100 |
| Category 3 | 400 | 400 |
| Category 4 | 1500 | 1500 |
| Category 5 | 1500 | 1500 |
FDA has identified a number of APIs that have secondary or tertiary amines and at risk for forming NDSRIs and has published an Updated Information | Recommended Acceptable Intake Limits for Nitrosamine Drug Substance-Related Impurities (NDSRIs)
The approach elaborated in the USFDA guidance is similar to a Q&A document published by EMA, except that for category 1, the AI limit is 26.5ng/day (in the EMA Q&A document for MA Holders and applicants for Category 1 the AI limit is 18ng/day). However the FDA guidance do not define criteria for a temporary AI (t-AI) or interim AI limit, and recommend manufacturers and applicants to contact agency when marketed products have levels of NDSRIs above the recommended AI Limits. The guidance also directs that where manufacturers and applicants have not considered NDSRI in their Nitrosamines risk assessment, it should be reevaluated within 3 months of publication of the guidance (by 1 November 2023).
EMA updates the Q&A for MA Holders
EMA explains in the new update dated 28 July 2023, that with new approaches for setting nitrosamines limits using the carcinogenic potency categorisation approach (CPCA), the approach for a universal temporary AI (t-AI) while a formal AI is established is no longer considered necessary and content under Q&A 21 is deleted. This removes the provision for adopting temporary AI (t-AI) of 178 ng/day (total nitrosamines), till a formal AI is established for newly identified N-Nitrosamines (as per the Q&A document dated 7 July 2023). The approach to be followed is to use of an interim limit as described in Q&A 22, based on less-than lifetime (LTL) approach where upto 12 months (13.3 x AI) and > 12months (6.7 x AI) limit shall be applied. However limit should not exceed 1.5 µg/day.
Leave a Comment
You must be logged in to post a comment.