FDA published the fiscal year FY 2024 GD
The GDUFA fee rates show steepest increase for DMF by 21% to $94,682 from $78,293.

Warning letters, 483s, Recalls, Import Alerts, Audit observations

The GDUFA fee rates show steepest increase for DMF by 21% to $94,682 from $78,293.

Intas facility was inspected by FDA investigators Jose E Melendez, Justin A Boyd, Pratik S

Lupin recalls 4179 boxes of Tydemy. Tydemy is Drospirenone, Ethinyl Estradiol & Levomefolate Calcium Tablets

Tarsus Pharmaceuticals Inc., in a statement announced FDA approval of Lotilaner ophthalmology solution 0.25% (XDEMVY™).
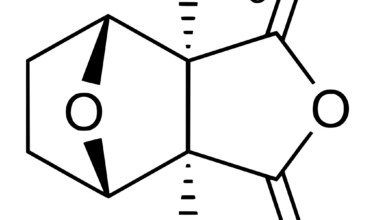
Verrica Pharmaceuticals Inc. is a dermatology therapeutics company developing medications for skin diseases requiring medical

EMA updates Q&A document on N-Nitrosamines. In the Rev 16 (dated 7 July 2023) limits

Centaur Pharmaceuticals, India issued Warning letter by USFDA. The Warning letter dated 5 June 2023

EMA, the European Commission (EC) and the Heads of Medicines Agencies (HMA) are phasing out