Temperature excursion in transport cause
Baxter initiated recall of one lot of Doxil (doxorubicin hydrochloride liposome injection) in U.S due

Warning letters, 483s, Recalls, Import Alerts, Audit observations

Baxter initiated recall of one lot of Doxil (doxorubicin hydrochloride liposome injection) in U.S due

SCYNEXIS recalled 2 lots of Brexafemme (ibrexafungerp) tablets in U.S. due to potential cross contamination

Hospira recalled in US one lot of 4.2% Sodium bicarbonate injection and one lot each

Florida based VistaPharm LLC is recalling in US one lot of ulcer treatment drug Sucralfate

Centaur India formulation facility at Pune, Maharashtra, India was issued Warning letter by FDA citing

Abbot recalls all batches of Digene Gel of all flavours (Mint, Orange, Mix fruit flavours)

Bottles of Digoxin Tablets, USP 0.125mg are incorrectly labelled and contain Digoxin Tablets USP, 0.25mg
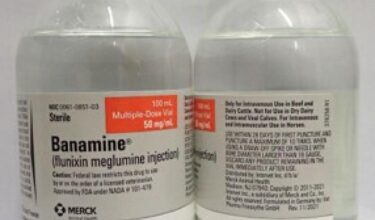
Flunixin meglumine is a potent, non-narcotic, nonsteroidal, analgesic agent with anti-inflammatory and antipyretic activity. It

The Warning letter cite Failure of Quality unit in ensuring cGMP compliance, Inadequate control over